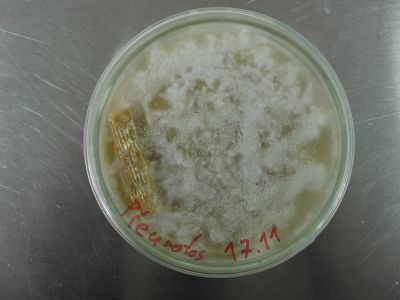
22. February
Oyster mushroom cloning:
SB samples are vigorous in growth and show no contamination.
SBD2 has been run on PDYA medium and shows most aggressive growth, it is growing on the top glass of the dish and breaking out.
Inoculated 2x6 petris dishes with King oyster obtained from Rewe german origin
and 2x6 with shiitake obtained from denns german origin ecological.
Both specimen were sprayed with ethanol prior to taking the tissue samples.
Incubation at 25°C.
Prepared jars with silicon for Liquid Culture technique according to Hippie3.
7. February
sample-----growth-----contamination-----next action-----comments
SAD1-------X-----------XXX-----------------discard--------------------
SAD2-------XX---------XXXX----------------discard--------------------
SAD3-------XX---------XX-------------------keep----------------------
SAD4-------XXX-------XX--------------------keep---------------------
SBD1-------XXXX------X---------------------run-------------brown metabolite
SBD2-------XXXXX----XX--------------------run-------------brown metabolite
SBD3-------XXXXX---------------------------run-------------brown metabolite
1. February
Moved Button mushroom storage slants to the fridge, as the wine cooler gets very hot when the sun is shinning on it.
Oyster mushroom cloning:
sample------growth-----contamination-----comments
SAD1--------------------XXX----------------------------
SAD2--------X-----------X-------------------------------
SAD3--------X-----------XX-----------------------------
SAD4--------X-----------XX-----------------------------
SBD1--------?-----------XXX-----------------unclear what is growing!
SBD2--------XX---------XX------------------------------
SBD3--------XXX----------------------------------------
31. January
Oyster mushroom cloning:
sample-----growth-----contamination-----comments
SAD1----------------------------------------------------
SAD2-------------------X--------------------------------
SAD3-------X-----------XX------------------------------
SAD4-------X-----------X-------------------------------
SBD1-------XX---------X---------------------(leapoff)
SBD2-------X-----------X-------------------------------
SBD3-------X-------------------------------------------
Placed Button Mushroom storage slants into cooling at 6°C.
30. January
One of the cold storage slants for brown button mushroom has developed a lustrous surface coating. The other slant is close to fully covering the surface,
Oyster mushroom cloning:
sample---growth---contamination----
-----------------------------------------
SAD1-----none-----none--------------
SAD2-----none-----none--------------
SAD3-----minor----none--------------
SAD4-----none-----mild--------------
SBD1-----very good----none---------
SBD2----minor------mild-------------
SBD3----good-------none
the temperature in the laboratory has dropped under 20° C, as somebody left the windows open, the cultures were moved into the incubator at 25,5°C.
28. January
Cloned 2 specimen of oyster mushrooms obtained from globus supermarket. The mushrooms are produced in Poland.
4 dishes were inoculated with specimen A. 3 dishes with specimen B. They are incubated at room temperature ( 23°C)
autoclaved the horrible jar of penicilium.
26. January
E³ shows aggressive leap off.
Inoculated two long-storage-slants with the Brown Button Mushroom mycelium (E²).
The old Pleurotus culture on cardboard gives mushrooms.
Moved it from cold storage to the windowsill.
24. January
Transferred rhyzomorphic mycelium to E³.
23. January
E² shows rhyzomorphic growth.
Blanks are clean.
18. January
E² shows excellent leap of.
Blanks are clean.
14. January
Poured and sterilized plates.
E² is growing rapidly.
Two blanks are incubated to see if there is any contamination introduced through bad sterile-technique.
12. January
Due to long illness all cultures are neglected:
-all Grain-masters are over-incubated, None is usable for further development.
Through poor air-circulation (no shaking) anaerobic conditions prevented the mycelium running. The mycelium itself looks healthy, but green molds are sharing the habitat.
The mycelium ranges in colour from white over purple to red.
The Button Mushroom cloning:
all dishes save E, are contaminated and the mycelium has died.
E sports cottony mycelium and it seems like primordia forming, there is no contamination.
The mycelium from E is transferred to another dish labelled E².
13. December
Button Mushroom cloning. dishes showing mycelial leap of on the medium: A. B. C. D. E. Dishes showing no myceliuml growth whatsoever: G.
the picture was taken on the 13th. (the date above the line should read 9.12 not 9.11) After the picture was taken I shock all G2 Masters. One of the G2A jarls lost its professional filter cotton ball. I employed questionable practices and replaced the cotton ball after extraction under the laminar flow hood, with a new one I soaked in ethanol. I separated the jar from the others and marked it with a red circle.
9. December
Grain 2 Masters running! Marked G2B.
G2A mycelium is getting a pinkish tone!
Inoculated 6 Potato Dextrose Agar dishes with Brown Button Mushroom
Method: cloning. Strain: commercial, Poland ( from the supermarket)
8. December
Inoculated 3 G2B.
7. December
sterilised the other 3G2
Two Pleurotus samples were dried out in their dishes in an attempt to let them form primordia, with they did, until they where hard and dead.
They might be of value to Suna, and her paper project, I keep them.
6. December
sterilised 3 of the G2 and inoculated with G1A.
I built an magnetic stirrer. It runs on 5 volts. Should be sufficient for now.
5.December
G1 Masters are running.
Autoclaving dark mutants and empty kimchi jar.
Prepared six G2 bottles according to Stamets (soaking overnight to germinate endospores)
3. December
Placed one of the G1 Masters in the incubator at 25°C.
1. December
Inoculated Grain Master G1. (2x 150g)
30. November
Prepared rye for grain master according to Stamets.
29. November
The kimchi jar shows no signs of growth, nor contamination. Nothing shows up under the microscope.
26.November
All Pleurotus samples look very promising. The F1 generation samples are thriving. No contamination at all. Very soon the production of spawn can begin. The peg sample is now all cottony mycelium. So no yeasts, just the look of them. Maybe there are more of this early stage structures in the sample, or it is minor contamination. The control blank shows no sign of contamination.
Inoculated on jar of LA-bacteria-medium with kirby and cabbage kimchi samples.
19. November
Pleurotus: all samples show good growth and no contamination. Cottony mycelium (typical for pleurotus ostreatus) is developing.
The peg jar looks strange though. I rolled the peg around in the dish on the 17th. Now the surface of the medium shows the tracks of this action. But the developing organism looks rather yeasty than mycelish.
17. November
sterilised and inoculated 3 petri dishes with Pleurotus strains from the peg on malt-medium. Transferred the original peg to one petri dish and kept one blank for controlling. All dishes are Potato-Dextrose-Agar-Medium.
the tupperware pleurotus caught on in growing, but still is very restrained.
16.November
Cooked and sterilised 1 Liter of Potato-Dextrose-Agar-Medium. Poured 5 petri-dishes, to be sterilised on friday.
Still no luminescence in vibr. phosp.
15. November
Pleurotus in malt-medium spawns cottony mycelium. The sample shows mild contamination.
the tupperware sample still is, if growing very slowly but shows no signs of contamination.
the temperature of the incubator is now 25.5°C
9. November
Discarded jar A of vibrio phosp. due to high contamination.
transferred on of the pleurotus pegs on malt medium. (Paul Stamets, The Mushroom Cultivator)
8. November
vibrio phosp. dish A shows signs of contamination, I assume due to autoclaving problems as mentioned above.
still no luminescence, I am beginning to suspect them to be dark mutants (Osamu Shimomura: Bioluminescence, Chemical Principles and Methods chapt. 2.7 p. 43 subchapt. Formation of dark mutants)
Pleurotus grow is, if, growing very slowly, planning to move on of the two pegs to another medium.
[[File:1590301.jpg|400px]]
supplementary: vibrio phosp. opened jar A and shock it vigorously under the laminar hood, to get oxygen to the bacteria, which should aid glowing. no effect. the sample has to be discarded anyway.
2.November
Pleurotus growth becomes apparent.
vibrio phosp. still no luminescence. maybe the concentration of the the autoinducer is still to low in the medium. (Osamu Shimomura: Bioluminescence, Chemical Principles and Methods chapt. 2.7 p. 42 subchapt. Auto induction)
Euglena seem to spin when there is not enough light for them, rather an otherwise. Which makes perfect sense (appetitive behaviour) but somehow I thought it to be the other way round.
1.November
Autoclaved two dishes filled with LA-Medium, one of the dishes filled up with water during the process, discarded and poured with leftover medium that has been in the autoclave, and inoculated with vibrio phosphoreum around 16.00.
they should start to glow in 12-24 hours.
31. October
starting to grow vibrio phosphoreum from a batch that was labelled Photobacterium phosp. and seemed to be inoculated somewhere in 2016. (the writing on the petri-dish was rendered unreadable)
cooked 100ml of LA-Medium and left it overnight in the fridge.
on plastic petri-dishes: I decided to use only glass-petri-dishes, as they give me the opportunity to sterilise medium inside the dishes, thus limiting to chances for contamination greatly. and they produce less waste, are to be recycled if broken, if not broken have a unlimited shelf life and usage time.
migrated the pleurotus from the plastic mushroom supermarket dish to a tupperware. no sign of growth.
25. October
inoculated cooked cardboard with Pleurotus pegs.