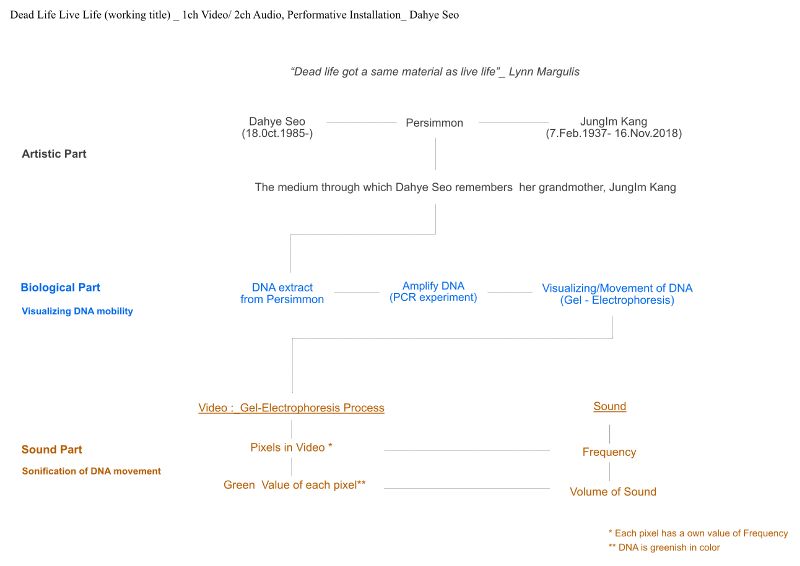
Dead Life Live Life (working title)
Dead Life Live Life is a sound installation work that sonifies the movement of DNA. This work was inspired by the poetic movement of DNA in electrophoresis experiments. Dead Life Live Life explores the materiality of DNA itself rather than genetic analysis, which is the general purpose of DNA. “Dead life got the same material as live life”, biologist Lynn Margulis said in an interview. This could be paraphrased as “Dead life got the same DNA as live life”. Dead Life Live Life poetically represents the connection between life and death by sonifying the DNA movement of an old persimmon tree planted in my grandmother's house.
Technical solution
1. Biological part
Instruction of DNA analysis
1) DNA Extraction of Persimmons
2) PCR Experiment (DNA amplification) / Gel electrophoresis (DNA movement & visualization) .
DNA of the persimmon is amplified through a PCR experiment. The amplified DNA is visualized in a gel electrophoresis experiment. DNA molecules dyed to respond to UV light glow and flow electromagnetically in an agar-gel chamber
2. Sound part
1) Sonification of DNA movementMax/Msp patch
Convert continuously changing number of RGB data (could be change to other data) of DNA movement animation to midi or frequency.
2) Sound Design
Work in Biolab
2024.11.16. Sat
Introduction
Gel Electrophoresis Exercise
I started by relearning how to use a micropipette to pick up where I left off a year ago. I tested two DNA ladders and a green stain that I had stored in the freezer to see if they were still functional.
Procedure
recipe
Agarosegel : 0.5g Agarose, 50 ml TBE Buffer, 2.5 µl DNA Stein green
TBE Buffer for cover gel : 50 ml TBE Buffer, 1.25 µl DNA Stein green
Gel Electrophoresis : 5V - 40 min -> 5V - 40 min -> 6V - 60 min (current 400 in every process)
The instructions say to electrophoresis at 5v for 40 min. Due to the short distance of DNA migration, I did 2 additional cycles.
Result
First Column: 1 kb DNA ladder
Last to first and second Columns: 100 bp DNA ladder
*DNA moves slow. Next time, reduce the percentage of agarose gel ?
How to set up the current for the gel electrophoresis? Does it impact the value of current for the movement of DNA?
ps. after around 50 hours. Still there remains the trace of the DNA movement.
2024.11.18. Mon
Introduction
The purpose of this experiment is to find out how DNA migrates when exposed to UV light throughout the whole gel electrophoresis process.
Procedure
A 100 bp DNA ladder was electrophoresed at 50 volts, 40 minutes.
* Note
1. Reused the gel from 2 days ago.
2. Did not put stain green in the TBE buffer covering the gel.
Result
When the UV was shined throughout the experiment, the DNA movement is very blurry compared to when it was not.
However, I am not sure if this is due to the UV light or the reuse of the gel or the lack of stain green.
2024.11.19. Tue
Introduction
DNA Extraction Exercise_ Kaki,Blueberry
Procedure
I followed the guide from Julian's workshop (see photo above)
Result
Blueberries were successful in extracting DNA, but kaki failed again.
2024.11.20. Wed
Introduction
DNA Extraction Exercise_ Kaki, Leaf
- I failed to extract Kaki's DNA in my experiment yesterday. This time i followed the guide from the result of research by Alessandro to extract Kaki's DNA.
- Alessandro's research results show that DNA is mainly extracted from the leaves of the persimmon. Since I can't get the leaves of the persimmon right now, I tried the experiment with the weeds on the roadside.
Procedure
-The DNA extraction experiment was performed following the guide of chat gpt for the persimmon and the existing instructions for the leaf.
To extract DNA from kaki (persimmon) fruit, you'll need about 3-4 fruits to get a sufficient amount of DNA for analysis. Here's a step-by-step procedure:
### Materials Needed:
- 3-4 kaki fruits
- 5g washing up liquid or hand soap
- 2g salt
- 100ml tap water
- 100ml ice-cold alcohol (isopropyl alcohol or ethanol)
- Knife and fork for cutting and mashing
- Beaker or large bowl
- Coffee filter or fine sieve
- Saucepan for hot water bath
- Ice water bath
### Procedure:
1. Preparation: Peel the kaki fruits and cut them into small chunks. Remove any seeds.
2. Mashing: Mash the fruit chunks thoroughly in a beaker or large bowl to break down the cells.
3. Extraction Buffer: Mix the washing up liquid, salt, and tap water in a separate container. Stir gently to avoid creating bubbles.
4. Mixing: Add the extraction buffer to the mashed fruit and mix well.
5. Incubation: Place the mixture in a hot water bath at 60°C for 15 minutes. Stir occasionally to ensure even heating.
6. Cooling: Transfer the mixture to an ice water bath and let it cool for 5 minutes.
7. Filtration: Filter the mixture through a coffee filter or fine sieve into a clean container. This will remove solid debris.
8. DNA Precipitation: Slowly pour ice-cold alcohol down the side of the container to form a layer on top of the filtered mixture. DNA will precipitate out as a white, jelly-like substance at the interface.
9. Collection: Use a medicine dropper or a hook made from thin wire to collect the DNA precipitate.
Result
Both the leaves and the persimmon failed to extract DNA. I was a little frustrated.
It seems like I need to extract DNA from the leaves persimmon tree.
But where do I get the leaves?
2024.11.21. Thu
Introduction
Various sample preparations for pcr and gel electrophoresis : DNA extraction of banana and blueberry.
Procedure
I followed the guide from Julian's workshop as usual
Result DNA extraction was successful for both bananas and blueberries. DNA extraction is particularly easy for bananas. Blueberries, like strawberries and raspberries, do not have a high DNA extraction rate compared to other berry types.
2024.11.23. Sat
Introduction
Exercise for DNA centrifugation and PCR Experiment
Procedure
I followed the instruction in Julian's workshop for setting up the centrifugation of DNA sample and PCR.
- Centrifuge setting: 8kg, 60 seconds
- PCR: see instructional photo above.
A total of five DNA samples were subjected to DNA amplification by PCR
- Label information
- B11: DNA of Blueberries (from 19.Nov)
- B12: DNA of Blueberries (from 19.Nov)
- B2: DNA of Blueberries (from 21.Nov)
- BA: DNA of Banana (from 21.Nov)
- K: Kaki pulp with soap, salt, hot water (from 20.Nov)
*sample B11, B12 are dirtier than B2
*So far no success to extract Kaki DNA. I used this time kaki pulp instead of DNA. It seems like there are no DNA in pulp, but at least I wanted experiment with pulp until Gel-electrophoresis just for double checking.
2024.11.26. Tue
Introduction
Extracting DNA from leaves using DNeasy Kit with supervising by Alessandro Volpato_ Since there are no leaves from persimmon trees, I experimented with leaves from other trees in Ilm park.
Procedure
The protocol below was followed.
https://youtu.be/c1WOBy5vSg8?si=ksFltpYkG1ytvugT
Result
I followed the protocol to extract DNA from the leaves, but I could not visually check if the DNA was extracted correctly. After the gel electrophoresis, i I will know if the DNA was extracted. �I will start electrophoresis as soon as the Stein green arrives.