m (Miga moved page GMU:BioArt/Crystals and cellular automata to GMU:BioArt WS15/Crystals and cellular automata) |
|||
| (18 intermediate revisions by 2 users not shown) | |||
| Line 12: | Line 12: | ||
<gallery> | <gallery> | ||
image:3-snowflakes.png | image:3-snowflakes.png| Snowflakes. Source: A New Kind of Science: Stephen Wolfram | ||
</gallery> | </gallery> | ||
==Crystals== | ==Crystals== | ||
=== structure === | === structure === | ||
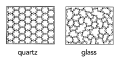
Crystal atom lattice with periodic order and glass having no periodic order of atoms | |||
<gallery> | |||
Image:2-what-is-glass2.png| Crystal and glass | |||
</gallery> | |||
more: [https://en.wikipedia.org/wiki/Crystal wikipedia] | |||
=== crystallization and cellular automata === | === crystallization and cellular automata === | ||
=== organigenic crystals and pearls === | === organigenic crystals and pearls === | ||
crystals could be also produced by organisms, eg shells produce pearls | |||
==Emergence of Creative Forms in Cellular Automata== | ==Emergence of Creative Forms in Cellular Automata== | ||
=== patterns: snowflakes, diamonds, and table salt === | === patterns: snowflakes, diamonds, and table salt === | ||
Stephen Wolfram introduced the idea that nature could be explained by simple rules, like cellular automata | |||
<gallery> | |||
image:3-snowflakes-ca.png| CA Snowflakes. Source: A New Kind of Science: Stephen Wolfram | |||
</gallery> | |||
=== cellular automata, fractals and universal computation === | === cellular automata, fractals and universal computation === | ||
=== game of life and CA demonstrating universal computation === | === game of life and CA demonstrating universal computation === | ||
For example, if tested, the rules of Game of Life, while evolving over generations, would look like a real fight for survival | |||
See for example [https://www.youtube.com/watch?v=C2vgICfQawE animated video] | |||
<br>more: [http://www.conwaylife.com/ Conway's "Game of Life"] | |||
To make things easier and to procure the results of the rules applied to the two-dimensional grid faster than one would on paper, a computer application may be used. In the application Golly46, a number of different rules can be applied and tested over the numerous generations. | |||
more: [http://golly.sourceforge.net/ Software: Golly] | |||
== Examples == | == Examples == | ||
=== Joe Davis, Bacterial Radio === | === Joe Davis, Bacterial Radio === | ||
[http://www.biofaction.com/synth-ethic/?p=44 Joe Davis, Bacterial Radio] | |||
=== Carsten Nicolai “Snow Noise” === | === Carsten Nicolai “Snow Noise” === | ||
[http://www.carstennicolai.de/?c=works&w=snow_noise Carsten Nicolai “Snow Noise”] | |||
=== Roger Hiorns, Seizure === | === Roger Hiorns, Seizure === | ||
=== Martin Howse and Johnatan Kemp | [http://www.theguardian.com/artanddesign/video/2013/jun/13/roger-hiorns-seizure-yorkshire-video Roger Hiorns, Seizure] | ||
=== Martin Howse and Johnatan Kemp “The Crystal World” === | |||
*[http://crystal.xxn.org.uk/wiki/doku.php Project: The Crystal World] | |||
*[http://www.metamute.org/editorial/articles/crystal-world Text: "the crystal world" By Martin Howse and Jonathan Kemp] | |||
*[http://www.metamute.org/editorial/articles/garden-earthly-delights Review: THE GARDEN OF EARTHLY DELIGHTS By Matthew Fuller] | |||
==Lab work (growing crystals)== | ==Lab work (growing crystals)== | ||
<gallery> | |||
Image:IMG_2561.png | |||
Image:IMG_2562.png | |||
Image:IMG_2563.png | |||
</gallery> | |||
---- | |||
'''Presentation on Salt crystals by Liese Endler''' | |||
In WS2014/15 I started working with Magnesium sulfate and Aluminum potassium sulfate. To get a saturated solution that will start growing crystals I used following receipts: | |||
Magnesium sulfate solution: | |||
mix salt and water in relation 1 to 3 | |||
e.g. 50g magnesium sulfate + 150g distilled water (warm) | |||
Aluminum potassium sulfate solution: | |||
mix salt and water in relation 1 to 2 | |||
50g aluminum potassium sulfate + 100g distilled water (warm) | |||
<gallery>Working_with_crystals1.JPG </gallery> | |||
My general observations on salt crystals so far: | |||
- once one small crystals started growing a lot more will follow (concept of the seed crystal) | |||
- when being exposed to music or other kinds of sonar waves / vibrations the crystals growth respond with different patterns | |||
- Magnesium sulfate-crystals are more easy to grow in a warm surrounding e.g. near the heater but not directly on it, sometimes it take a long time until the growing starts depending on the location of the jar the solution is in and the saturation | |||
- dusk, dirt or small particles of metal( e.g. from the metal spoon that one can use to mix the solution ) can interfere with the growing process | |||
- if you want to grow a big crystal its important to separate the one that you like the most from the others and put it into a fresh prepared solution that is cold | |||
<gallery> Alauncrystal_1.JPG </gallery> | |||
Exercise for the class: | |||
take a drop of magnesium sulfate-solution (MgSO4+H20) onto a microscope slide (Objektträger) | |||
try to work as clean as possible and take care the the drop is not full of dirt or dust. Wait a bit and than start to observe the drop through a microscope | |||
If you are a bit patient you can see the crystal growth. | |||
The salt molecules form straight lines and form solid patterns that remind of an icy surface. | |||
Some of the footage that I shot while observing crystaline structures and crystal growth under a microscope was used for a the fulldome-project | |||
[https://vimeo.com/148895787 "Crystal Cave"] | |||
Latest revision as of 15:52, 10 October 2017
Life-like Processes in Inorganic Systems
planets
Despite Miller's definition of living systems, their hierarchization might appear interesting at the levels introduced, which include atoms, organizations, and supranational systems. The definition also proposes that there is a non-living system on both sides of the hierarchy. On one side, there are inorganic molecules and, on the other side, inorganic planets and galaxies that demonstrate self-organized processes.
More: Miller, J. G. (1982). “The earth as a system.”
chemical elements and chemical compounds
For example, carbon itself, in the form of a crystal, is considered to be inorganic, but carbon as one element of some compound, wherein part of it is, for example, water, is considered necessary element for forming organic matter (consider alcohol, C2H6O, or methane, CH4). The complexity of defining living systems suggests that, even in the sciences, the border between the living and non-living varies depending on the context, and, therefore, it might be that the concept of life in the context of the human-machine distinction should be approached differently.
crystals: snowflakes, diamonds, and table salt
According to Miller's definition, self-organization emerging from interaction between matter and energy is a natural feature within living systems that lets them survive and continue to propagate. However, if only matter and energy were considered, self-organization would not be an exception in inorganic systems. On the contrary, growing crystals, including snowflakes, diamonds, and table salt, demonstrate life-like and self-organized processes. Life-like processes might be observed in turbulent flows, such as cigarette smoke, streams of moving vehicles, or oceanic currents. Biological evolution also suggests that organic forms evolved from inorganic forms, so, self-organized processes driven by matter and energy, as introduced by Miller, should not be considered a feature of organic nature alone.
Crystals
structure
Crystal atom lattice with periodic order and glass having no periodic order of atoms
more: wikipedia
crystallization and cellular automata
organigenic crystals and pearls
crystals could be also produced by organisms, eg shells produce pearls
Emergence of Creative Forms in Cellular Automata
patterns: snowflakes, diamonds, and table salt
Stephen Wolfram introduced the idea that nature could be explained by simple rules, like cellular automata
cellular automata, fractals and universal computation
game of life and CA demonstrating universal computation
For example, if tested, the rules of Game of Life, while evolving over generations, would look like a real fight for survival
See for example animated video
more: Conway's "Game of Life"
To make things easier and to procure the results of the rules applied to the two-dimensional grid faster than one would on paper, a computer application may be used. In the application Golly46, a number of different rules can be applied and tested over the numerous generations.
more: Software: Golly
Examples
Joe Davis, Bacterial Radio
Carsten Nicolai “Snow Noise”
Roger Hiorns, Seizure
Martin Howse and Johnatan Kemp “The Crystal World”
Lab work (growing crystals)
Presentation on Salt crystals by Liese Endler
In WS2014/15 I started working with Magnesium sulfate and Aluminum potassium sulfate. To get a saturated solution that will start growing crystals I used following receipts:
Magnesium sulfate solution: mix salt and water in relation 1 to 3 e.g. 50g magnesium sulfate + 150g distilled water (warm)
Aluminum potassium sulfate solution: mix salt and water in relation 1 to 2 50g aluminum potassium sulfate + 100g distilled water (warm)
My general observations on salt crystals so far: - once one small crystals started growing a lot more will follow (concept of the seed crystal) - when being exposed to music or other kinds of sonar waves / vibrations the crystals growth respond with different patterns - Magnesium sulfate-crystals are more easy to grow in a warm surrounding e.g. near the heater but not directly on it, sometimes it take a long time until the growing starts depending on the location of the jar the solution is in and the saturation - dusk, dirt or small particles of metal( e.g. from the metal spoon that one can use to mix the solution ) can interfere with the growing process - if you want to grow a big crystal its important to separate the one that you like the most from the others and put it into a fresh prepared solution that is cold
Exercise for the class: take a drop of magnesium sulfate-solution (MgSO4+H20) onto a microscope slide (Objektträger) try to work as clean as possible and take care the the drop is not full of dirt or dust. Wait a bit and than start to observe the drop through a microscope If you are a bit patient you can see the crystal growth. The salt molecules form straight lines and form solid patterns that remind of an icy surface.
Some of the footage that I shot while observing crystaline structures and crystal growth under a microscope was used for a the fulldome-project "Crystal Cave"