No edit summary |
No edit summary |
||
| Line 15: | Line 15: | ||
==References== | ==References== | ||
* https://ellieirons.com/projects/two-meadows/ | * https://ellieirons.com/projects/two-meadows/ | ||
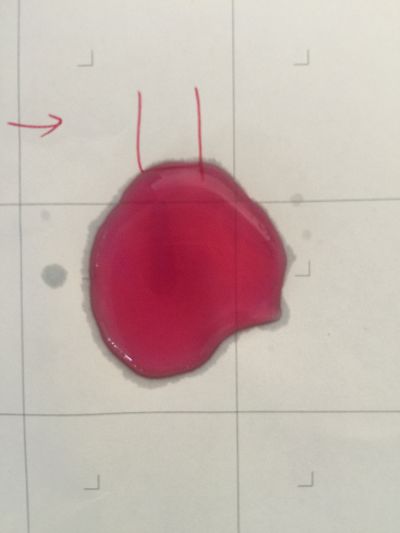
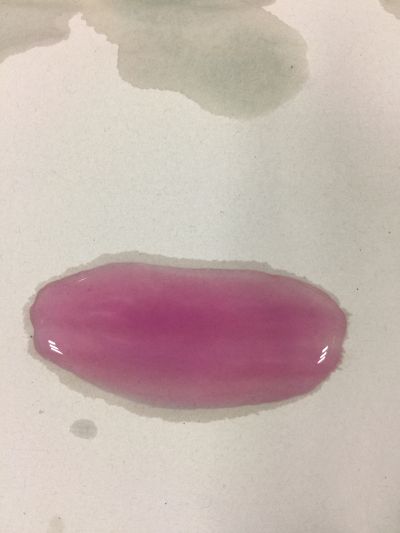
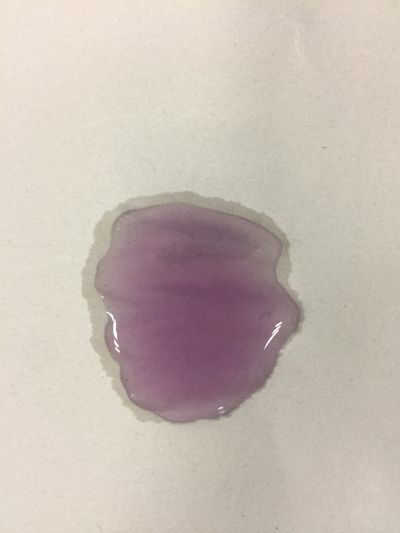
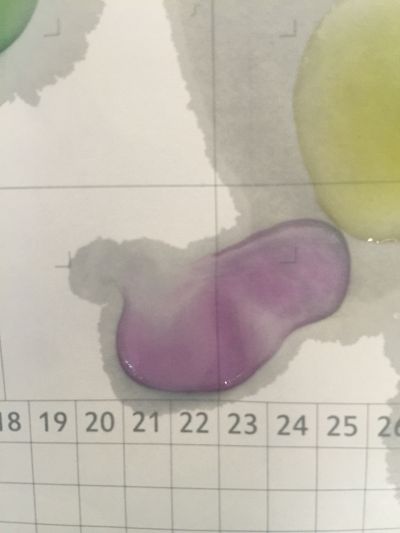
I use red cabbage to make pH indicator. The pH solution from 2 to 13. | I use red cabbage to make pH indicator. The pH solution from 2 to 13. | ||
The red cabbage solution | |||
[[File:WechatIMG440.jpeg|400px]] | [[File:WechatIMG440.jpeg|400px]] | ||
| Line 58: | Line 61: | ||
Next step: | Next step: | ||
1. Rate of colour change | 1. Rate of colour change | ||
2. Reversibility of colour change | 2. Reversibility of colour change | ||
Revision as of 16:22, 10 January 2018
References
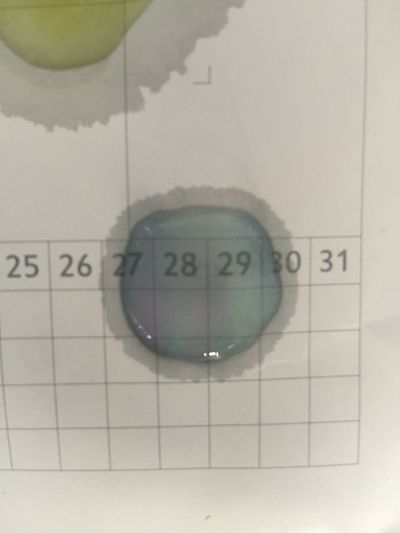
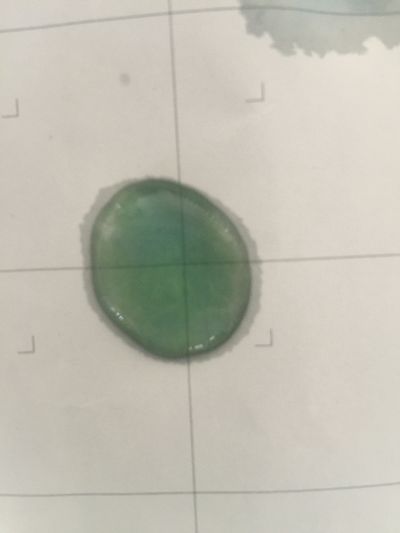
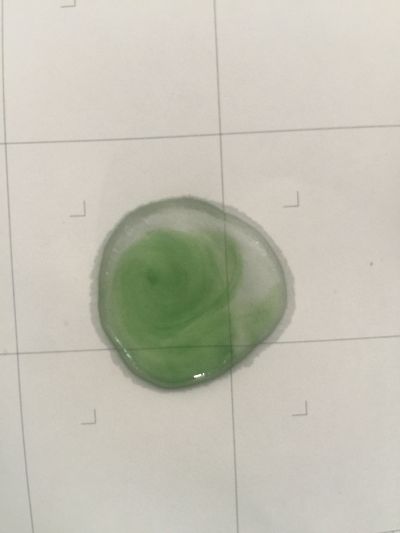
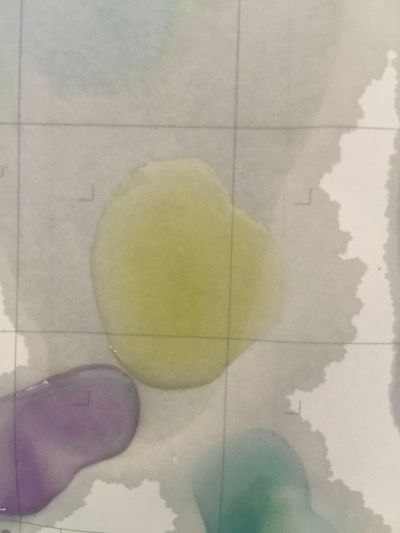
I use red cabbage to make pH indicator. The pH solution from 2 to 13.
The red cabbage solution
1. pH=2 100ml distilled water + 1ml HCL
2. pH=4 100ml distilled water + 0.1g apple acid
3. pH=5.5 100ml distilled water + 2g H3BO3(Boric acid)
4. pH=7 100ml distilled water
5. pH=8 100ml distilled water + 0.5g NaHCO3
6. pH=11 700ml distilled water + 1ml NaOH
7. pH=12 250ml distilled water + 1ml NaOH
8. pH=13 100ml distilled water + 1ml NaOH
after 5 minutes change to yellow
Next step:
1. Rate of colour change
2. Reversibility of colour change