No edit summary |
No edit summary |
||
| Line 12: | Line 12: | ||
[[File:12.6 bio-lab first idea.007.jpeg|400px]] | [[File:12.6 bio-lab first idea.007.jpeg|400px]] | ||
[[:File:12.6 bio-lab first idea.pdf]] | |||
==References== | ==References== | ||
Revision as of 16:25, 10 January 2018
References
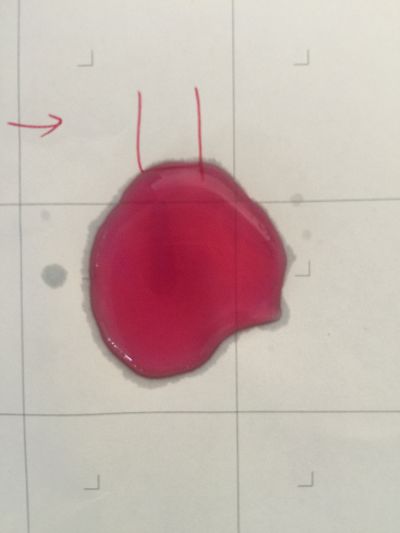
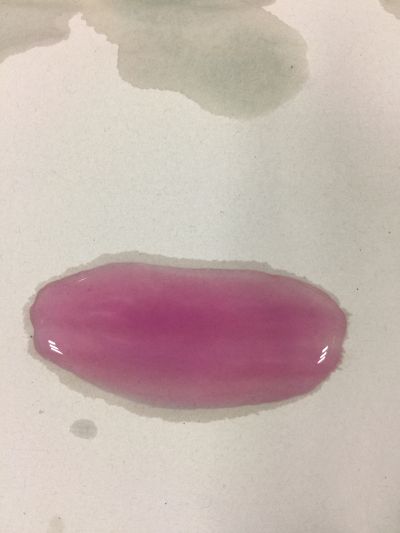
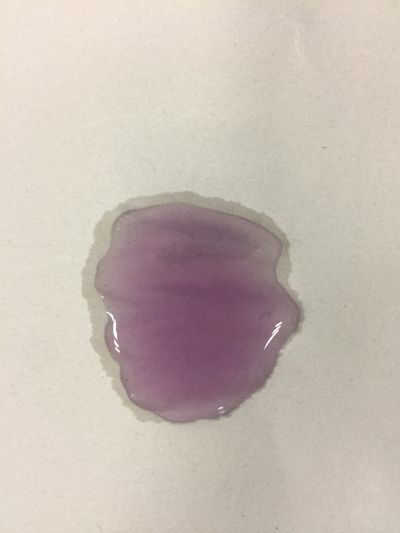
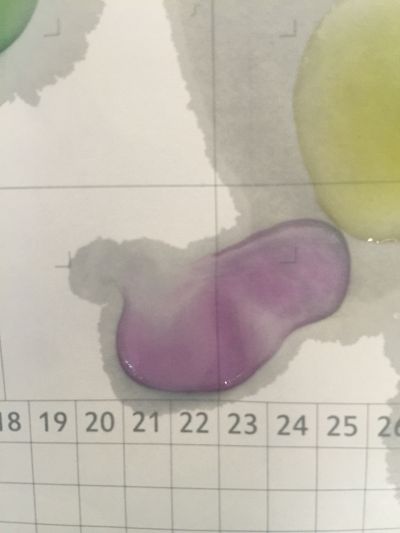
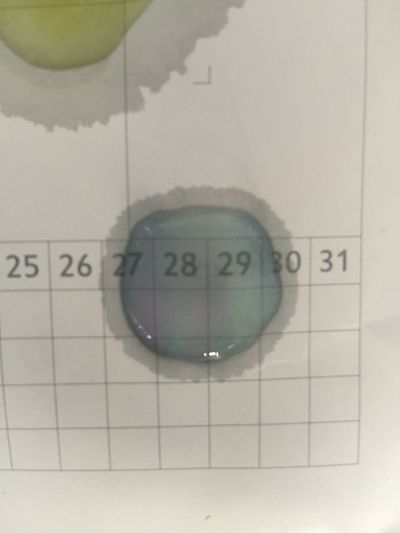
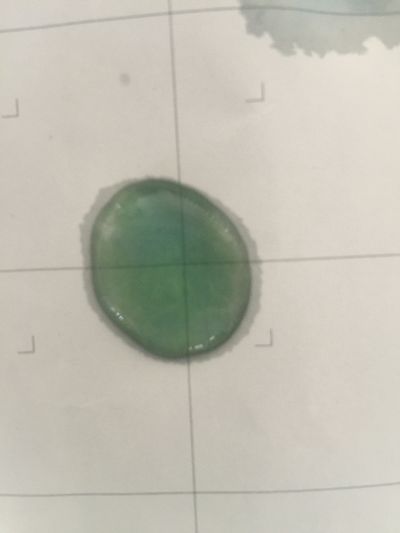
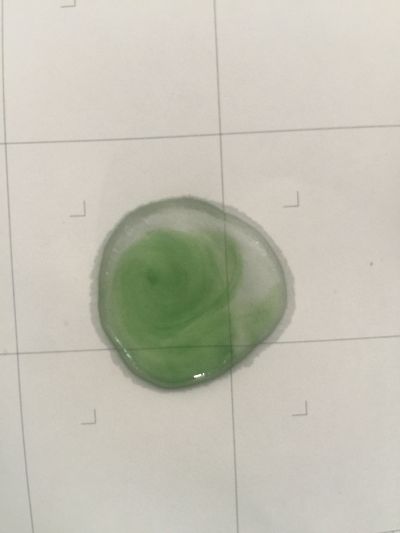
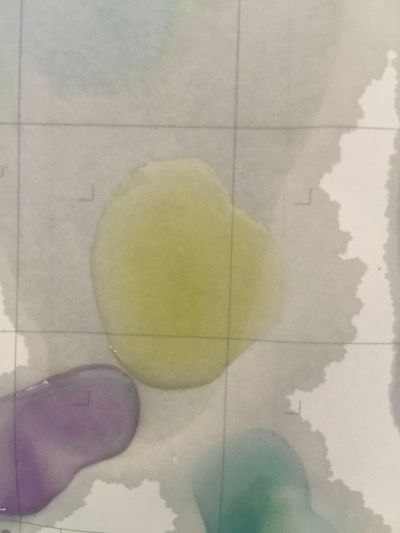
I use red cabbage to make pH indicator. The pH solution from 2 to 13.
The red cabbage solution
1. pH=2 100ml distilled water + 1ml HCL
2. pH=4 100ml distilled water + 0.1g apple acid
3. pH=5.5 100ml distilled water + 2g H3BO3(Boric acid)
4. pH=7 100ml distilled water
5. pH=8 100ml distilled water + 0.5g NaHCO3
6. pH=11 700ml distilled water + 1ml NaOH
7. pH=12 250ml distilled water + 1ml NaOH
8. pH=13 100ml distilled water + 1ml NaOH
after 5 minutes change to yellow
Next step:
1. Rate of colour change
2. Reversibility of colour change