No edit summary Tags: Manual revert Visual edit |
No edit summary |
||
| (24 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
=== '''Dead Life Live Life''' === | === '''Dead Life Live Life''' === | ||
==== Description ==== | |||
<nowiki>*</nowiki> This project was started but not completed in SS 2023 and will be continued this semester. | <nowiki>*</nowiki> This project was started but not completed in SS 2023 and will be continued this semester. | ||
| Line 5: | Line 7: | ||
[[File:Dlll 1.001.jpg|center|frameless|800x800px]] | [[File:Dlll 1.001.jpg|center|frameless|800x800px]] | ||
[[File:Dlll 2..jpg|center|frameless|800x800px]] | [[File:Dlll 2..jpg|center|frameless|800x800px]] | ||
[[File:Diagram Dead LifeLiveLife.jpg|center|thumb|800x800px]] | |||
==== Technical solution ==== | ==== Technical solution ==== | ||
| Line 41: | Line 45: | ||
2) Sound Design | 2) Sound Design | ||
==== Work in Biolab ==== | |||
===== '''2024.11.16. Sat''' ===== | |||
<u>Introduction</u> | |||
Gel Electrophoresis Exercise | |||
I started by relearning how to use a micropipette to pick up where I left off a year ago. I tested two DNA ladders and a green stain that I had stored in the freezer to see if they were still functional. | |||
<u>Procedure</u> | |||
''recipe'' | |||
Agarosegel : 0.5g Agarose, 50 ml TBE Buffer, 2.5 µl DNA Stein green | |||
TBE Buffer for cover gel : 50 ml TBE Buffer, 1.25 µl DNA Stein green | |||
Gel Electrophoresis : 5V - 40 min -> 5V - 40 min -> 6V - 60 min (current 400 in every process) | |||
The instructions say to electrophoresis at 5v for 40 min. Due to the short distance of DNA migration, I did 2 additional cycles. | |||
<gallery> | |||
File:DNA ladder1.jpg | |||
File:DNA Ladder2.jpg | |||
File:Prepare GelElectrophoresis.jpg | |||
File:Gel electrophoresis1.jpg | |||
File:GelElectrophoresis.jpg | |||
File:Note.jpg | |||
</gallery> | |||
<u>Result</u> | |||
First Column: 1 kb DNA ladder | |||
Last to first and second Columns: 100 bp DNA ladder <gallery> | |||
File:First round 5V-40min.jpg | |||
File:2round 5v-40min.jpg | |||
File:3round 6v-60min.jpg | |||
</gallery> | |||
<nowiki>*</nowiki>DNA moves slow. Next time, reduce the percentage of agarose gel ? | |||
How to set up the current for the gel electrophoresis? Does it impact the value of current for the movement of DNA? | |||
<small>ps. after around 50 hours. Still there remains the trace of the DNA movement.</small> | |||
<gallery>File:After 50hours gelElectrophoresis.jpg</gallery> | |||
===== ''' 2024.11.18. Mon''' ===== | |||
<u>Introduction</u> | |||
The purpose of this experiment is to find out how DNA migrates when exposed to UV light throughout the whole gel electrophoresis process. | |||
<u>Procedure</u> | |||
A 100 bp DNA ladder was electrophoresed at 50 volts, 40 minutes. | |||
<small>* Note</small> | |||
<small>1. Reused the gel from 2 days ago.</small> | |||
<small>2. Did not put stain green in the TBE buffer covering the gel.</small> | |||
<u>Result</u> | |||
When the UV was shined throughout the experiment, the DNA movement is very blurry compared to when it was not. | |||
However, I am not sure if this is due to the UV light or the reuse of the gel or the lack of stain green. | |||
<gallery> File:DNALadder UV lighting.jpg </gallery> | |||
Latest revision as of 22:32, 18 November 2024
Dead Life Live Life
Description
* This project was started but not completed in SS 2023 and will be continued this semester.
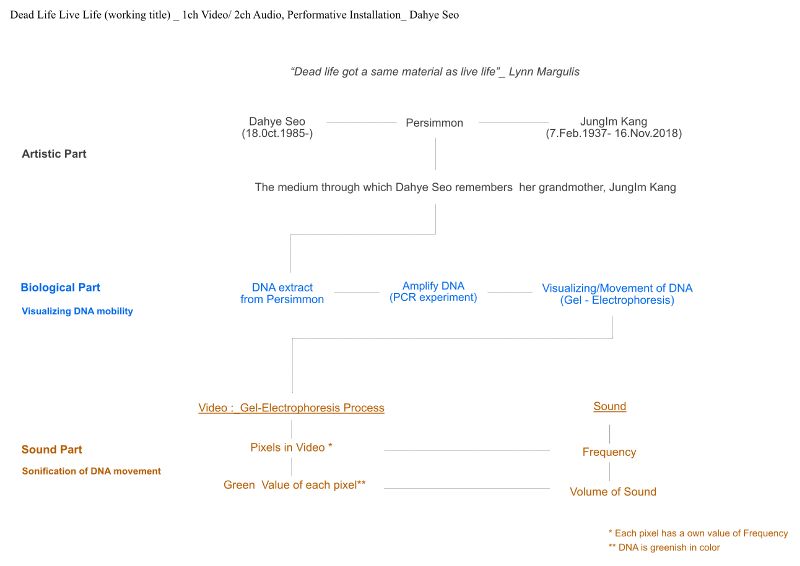
Dead Life Live Life is a sound installation work that sonifies the movement of DNA. This work was inspired by the poetic movement of DNA in electrophoresis experiments. Dead Life Live Life explores the materiality of DNA itself rather than genetic analysis, which is the general purpose of DNA. “Dead life got the same material as live life”, biologist Lynn Margulis said in an interview. This could be paraphrased as “Dead life got the same DNA as live life”. Dead Life Live Life poetically represents the connection between life and death by sonifying the DNA movement of an old persimmon tree planted in my grandmother's house.
Technical solution
1. Biological part
Instruction of DNA analysis
1) DNA Extraction of Persimmons
2) PCR Experiment (DNA amplification) / Gel electrophoresis (DNA movement & visualization) .
DNA of the persimmon is amplified through a PCR experiment. The amplified DNA is visualized in a gel electrophoresis experiment. DNA molecules dyed to respond to UV light glow and flow electromagnetically in an agar-gel chamber
2. Sound part
1) Sonification of DNA movementMax/Msp patch
Convert continuously changing number of RGB data (could be change to other data) of DNA movement animation to midi or frequency.
2) Sound Design
Work in Biolab
2024.11.16. Sat
Introduction
Gel Electrophoresis Exercise
I started by relearning how to use a micropipette to pick up where I left off a year ago. I tested two DNA ladders and a green stain that I had stored in the freezer to see if they were still functional.
Procedure
recipe
Agarosegel : 0.5g Agarose, 50 ml TBE Buffer, 2.5 µl DNA Stein green
TBE Buffer for cover gel : 50 ml TBE Buffer, 1.25 µl DNA Stein green
Gel Electrophoresis : 5V - 40 min -> 5V - 40 min -> 6V - 60 min (current 400 in every process)
The instructions say to electrophoresis at 5v for 40 min. Due to the short distance of DNA migration, I did 2 additional cycles.
Result
First Column: 1 kb DNA ladder
Last to first and second Columns: 100 bp DNA ladder
*DNA moves slow. Next time, reduce the percentage of agarose gel ?
How to set up the current for the gel electrophoresis? Does it impact the value of current for the movement of DNA?
ps. after around 50 hours. Still there remains the trace of the DNA movement.
2024.11.18. Mon
Introduction
The purpose of this experiment is to find out how DNA migrates when exposed to UV light throughout the whole gel electrophoresis process.
Procedure
A 100 bp DNA ladder was electrophoresed at 50 volts, 40 minutes.
* Note
1. Reused the gel from 2 days ago.
2. Did not put stain green in the TBE buffer covering the gel.
Result
When the UV was shined throughout the experiment, the DNA movement is very blurry compared to when it was not.
However, I am not sure if this is due to the UV light or the reuse of the gel or the lack of stain green.