No edit summary |
|||
| Line 1: | Line 1: | ||
== Using red cabbage solution to check coffee pH level == | == Using red cabbage solution as an indicator to check coffee pH level == | ||
1. '''Introduction''' | |||
1.1 red cabbage | |||
"Red cabbage is rich in a number of bioactive substances, including anthocyanins"[Wiczkowski, 2012]. | |||
1.2 anthosynin | |||
2.'''Idea''' | |||
3. '''Methodology''' | |||
3.1. Making red cabbage indicator | |||
4. '''Discussion''' | |||
5. '''References''' | |||
Revision as of 10:43, 31 January 2018
Using red cabbage solution as an indicator to check coffee pH level
1. Introduction 1.1 red cabbage "Red cabbage is rich in a number of bioactive substances, including anthocyanins"[Wiczkowski, 2012]. 1.2 anthosynin
2.Idea
3. Methodology 3.1. Making red cabbage indicator
4. Discussion
5. References
File:12.6 bio-lab first idea.pdf
The food we eat every day has gone through a series of procedures before it ever reaches our stomachs. From the beginning of planting, fertilizing, harvesting, to transporting, distributing, and cooking, each procedure involves numerous engineering works and scientific knowledge, each has contributed to the final quality of the food, and each has contributed to the final health of our body. What I focus is when food or drinks mixed with each other, is it still the original one?
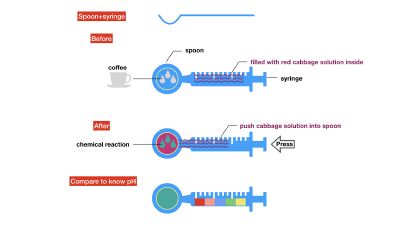
We drink coffee, mixed milk and sugar or curd. Many people have juicer, mixed kinds of fruits together, maybe it has a good flavour, but is it really good for your health? As we know, acid/Alkaline is very important to our health and the alkaline environment is good to our health, how can we know the pH of our drinks in a safe, readable, even edible way? I want to use red cabbage extract solution to know pH of different drinks, I can see changes of colour directly based on the chemical reaction and though comparing with pre-testing I have done, I can know pH of drinks easily. The sketch of pH-testing is
12.12.2017
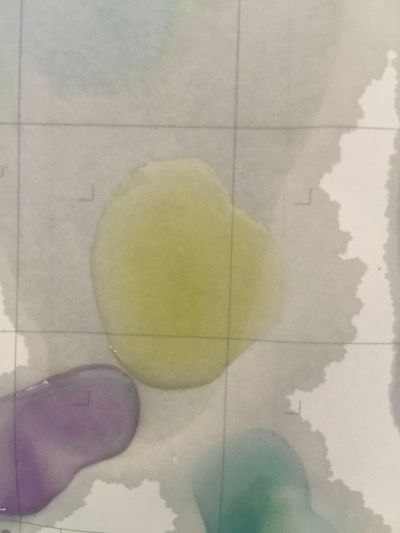
I use red cabbage to make pH indicator. The pH solution from 2 to 13.
The red cabbage solution
1. pH=2 100ml distilled water + 1ml HCL
2. pH=4 100ml distilled water + 0.1g apple acid
3. pH=5.5 100ml distilled water + 2g H3BO3(Boric acid)
4. pH=7 100ml distilled water
5. pH=8 100ml distilled water + 0.5g NaHCO3
6. pH=11 700ml distilled water + 1ml NaOH
7. pH=12 250ml distilled water + 1ml NaOH
8. pH=13 100ml distilled water + 1ml NaOH
after 5 minutes change to yellow
Furthur steps:
1. Rate of colour change
2. Reversibility of colour change
3. Using different pH level solutions react with the organic stuff (which has anthocyanin), see the result
17.1.2018
However, there’s a problem which I’m not sure: If pH has a direct relationship with health. I read some report and paper, there is a view: alkaline body environment is good for health, but it can not be said alkaline food leads to the alkaline environment/condition, it is complex medical problem. And also, this is a website of Acid/Alkaline Food Chart: http://www.phmiracleliving.com/t-food-chart.aspx And I find some healthy medicines which are used to adjust pH, the main ingredients are moderately or highly alkaline food. I am confused with it.
What I will do on 17th:
1. test just for visual and take picture:orange/lemon/onion/green onion/potato...
2. help to know pH level of drinkings
original pH
mixed pH (milk/coffee/tea/gouqi/sugar) (And is there any pH difference belongs to temperature? hot and cold )
question: how to deal with the thing insoluble in water, for instance, oil, cosmetic...
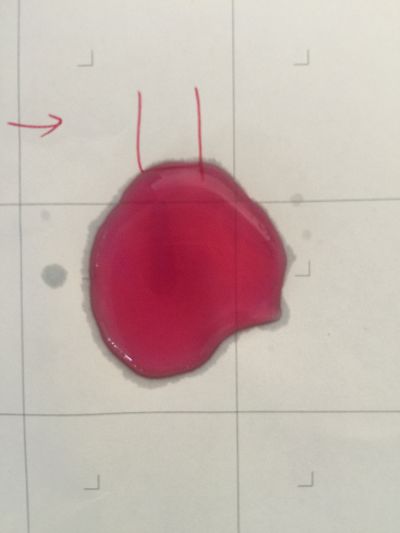
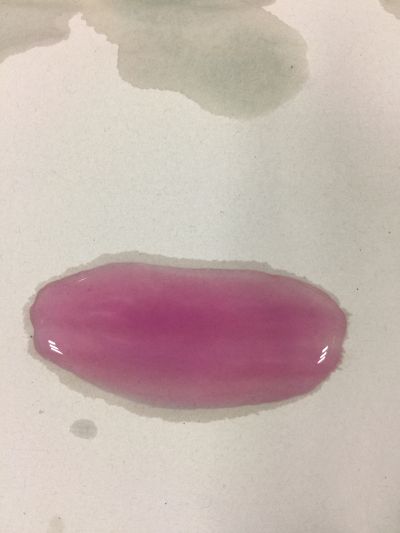
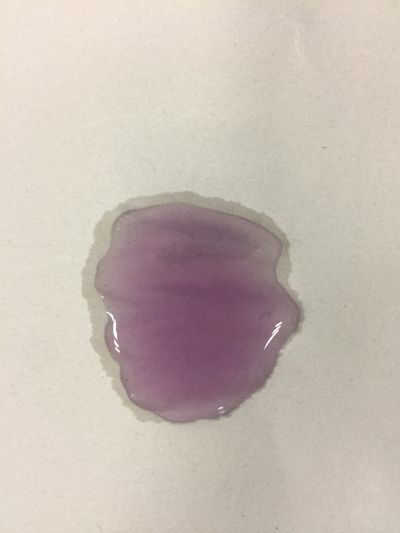
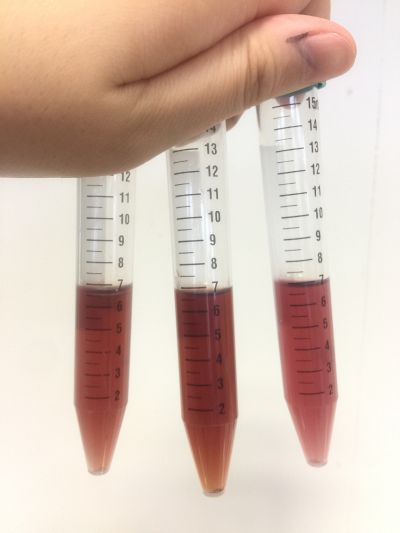
After the work of 17th, I decided to focus on coffee not kinds of drinks to do the experiment. Because it is hard to judge pH level with one colour measurement. I made 3 solutions with different proportions of red cabbage, distilled water and expresso coffee. 1. 5ml red cabbage solution + 1ml distilled water + 1ml coffee 2. 5ml red cabbage solution + 0.5ml distilled water + 1.5ml coffee 3. 5ml red cabbage solution + 1.5ml distilled water + 0.5ml coffee As you can see on follow picture, the coulour results are not obvious. The
19.1.2018
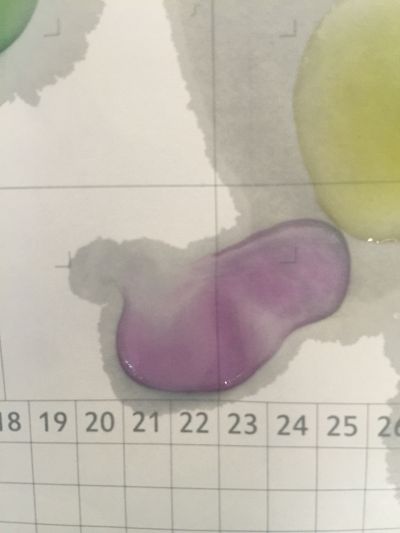
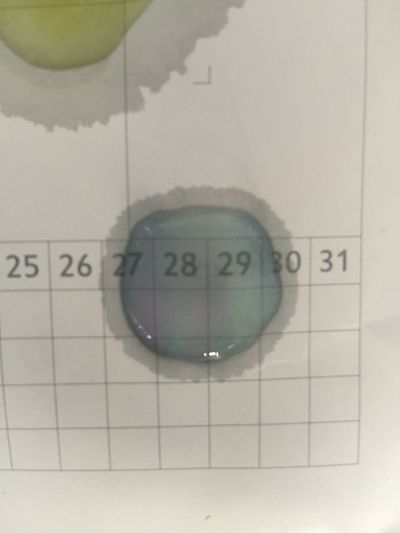
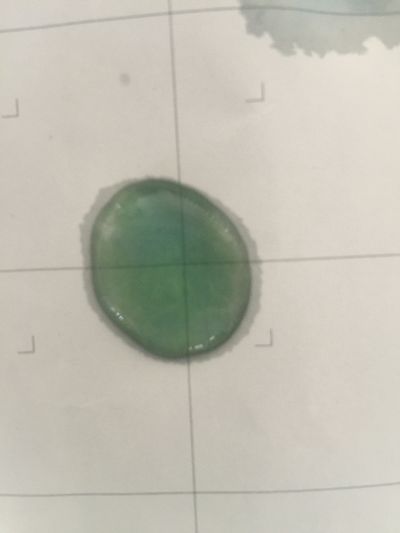
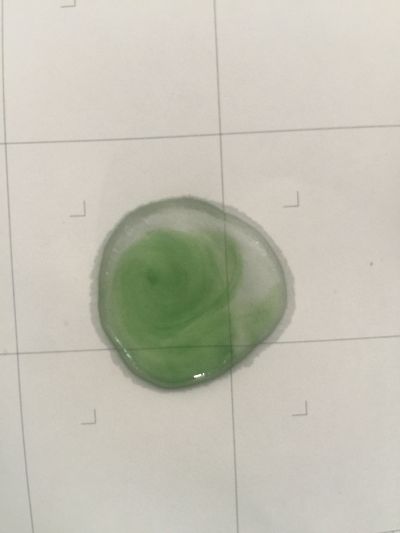
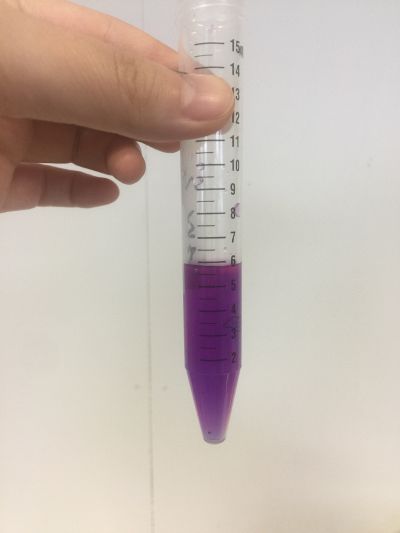
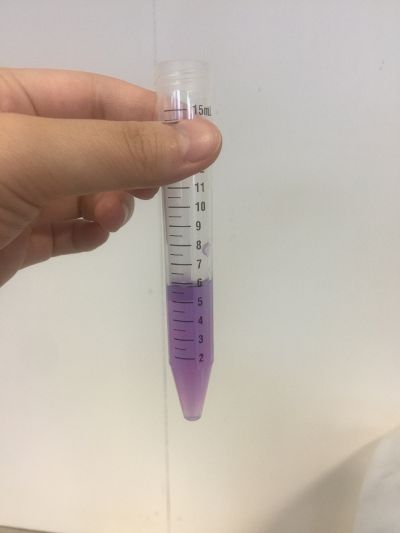
I did the experiment of reversibility of colour change.
5ml red cabbage solution + 2ml pH=2 + 2ml pH=9
5ml red cabbage solution + 2ml pH=2 + 2ml pH=11
5ml red cabbage solution + 2ml pH=2 + 2ml pH=13
5ml red cabbage solution + 2ml pH=4 + 2ml pH=9
And then I use reversibility of colour change to make the colour of coffee more obviously. pH of expresso coffee is around 6, I add solution (pH=9) into the original solution 1/2/3, the colour changing is more obviously.
However, when I change another kind of coffee and do the experiment in the same way, the colour change cannot be seen obviously. Maybe because the different ingredients of coffee.