Reaching for sunlight
Winter can be a rough time for people living in our lines of latitude. The sun is often hid by clouds, we become more tired and despondent. People use to substitute their lack of sunlight by traveling to warm places or taking Vitamin D. They start running after the last sunbeams because they know the impact of being without it. This reminded me of Euglena, the microorganism that is attracted by light. Do we share a similarity in this way?
By working on my experiments I wondered how I can connect this idea with my previous work. I thought about building a connection between humans reaching for light and the organisms that do the same. My first achievement is an Euglena painted picture which makes my thoughts visible.
Project
My project which I try to implement will be a picture made of Euglenas. If it is possible to move the organisms to places and in shapes I want (and eventuelly create contrasts) this should be realizable. But first I need to find out which options I have and where the borders of this idea are. The following experiments and the documentation of my work shall give an overview. In case of success the outcome will be a kind of installation or at least a piece which lasts for only a few minutes.
Research and Ideas
Interesting projects which mix art and microbiology by Mellissa Fisher:
What fascinates me most about Euglena gracilis is its ability to use photosynthesis or rather the need of this capability to survive.
Photosynthesis means that the organism converts light energy into chemical energy to power its energy balance. Euglena subsist heterotrophic and autotrophic; besides the process of photosynthesis (which is used by plants) it takes nourishment like animals as well. Euglena gracilis is a mixotrophic microorganism.
Besides that, it is able to recognize the stimulus of light (phototaxis). This function helps Euglena to move towards light sources to always assure the facility to use photosynthesis. [1]
During my researches I read the documentation of a project of a former student. She did a couple of experiments which included a tube wrapped with red foil to create a red light source. The result was that the organisms in the tube piled up on the only transparent place without foil because they don't recognize red light.
This made me think of several questions:
- What happens when the whole tube is wrapped with foil and there is only red light? What will the Euglena's concentration be like?
- How do Euglenas react to other colors?
- Is there a color they prefer?
- Can I create contrasts in the concentration by using different colors?
Furthermore, I wondered if it is possible to work creatively with Euglena’s phototaxis. As we can see, it always moves towards the light, so how about taking control over that?
- If I cover a glass receptacle with a dark medium, let’s say black, nontransparent paper, and cut out a shape to let light in, will the organisms accumulate there and fill this shape?
- Is it possible to arrange Euglenas in forms I want them to be?
Preparation
First of all I cooked a medium for Euglena which should promote the reproduction. It contains specific nutrients. Combined with a light position, e.g. at the window or in front of a lamp, they should accrete fast. Those microorganisms cannot be seen with the naked eye but if the population increases, the medium becomes greenish. This is my target goal because this is the state I want to work with.
A small microscope is my tool to observe Euglena. It has no light but a tiny mirror, so I use a flashlight to operate the microscope.
For having an overview of my work and making sure that I can work from home because of the not predictable Covid semester I build a little Euglena Workstation. It includes enough space to place all my tools and do experiments. Also, I can easily move it and position it to places which are reached by the sunlight to optimize Euglena’s growing.
Experiments
Experiment 1
How can I cultivate Euglena faster?
For all the experiments I want to do I need a huge amount of the microorganisms. After observing the medium I already cooked for about three weeks without noticing any change, I wanted to try out something new. I researched some more advices and tips to speed up the reproduction.
Location
- A room temperature of about 21 to 23 degrees Celsius is ideal for growing Euglena. So this is going to be the new climate of my room.
- Euglena need light, but no direct solar radiation. I chose a suitable window which fulfills those conditions.
- For making photosynthesis, every visible wavelength in the electromagnetic spectrum of light is utilized. UV-A light has a positive effect on the growing of the chloroplasts while UV-B light can slow down photosynthesis. t is not required in high amounts, so I will waive to use a UV lamp. The light Euglena gets naturally should be enough. [2]
Nutrition
- Euglena is not able to produce vitamin B12 by itself. The absorption occurs by the phagocytosis. This lack can be compensated with a tiny piece of hard cheese or some wheat grain.
- Because CO2 is required for the process of photosynthesis, it is recommended to add some stale mineral water from time to time. I will also use CO2 fertilizer for aquarium plants to see if this works as well. [3]
Initial state
- Before starting the experiment I checked under the microscope whether my Euglena are still alive. In all three tubes (two with the medium I cooked and one with the „pure“ Euglena I got for my work) they are moving.
Setup
I prepared six different vessels:
- Tube 1 - unchanged Euglena medium; hard cheese
- Tube 2 - unchanged Euglena medium; liquid fertilizer
- Tube 3 - distilled water; hard cheese; liquid fertilizer; CO2 fertilizer once per week
- Tube 4 - distilled water; hard cheese; liquid fertilizer
- Erlenmeyer flask - boiled water; hard cheese; liquid fertilizer; CO2 fertilizer once per week
- Beaker glass - boiled water; hard cheese; liquid fertilizer
I varied the consistence of the mixtures to see which differences I can observe and which of them is the best medium for Euglena.
Expactation
I guess that the nutrients I use this time work better for Euglena. During my researches I saw that various biology pages recommend the use of hard cheese and the addition of CO2. The tubes in which I am going to add CO2 will probably show a better growing of Euglena. Furthermore I think that in the tubes with the medium I have already made, the growing will be a bit faster because those Euglenas already got some nutrients; the pure Euglenas did not.
Implementation
Day 0 - preparing the vessels
Day 2 - recognizing something green settled on the ground of tube 1
Day 3 - adding CO2 to tube 3 and the Erlenmeyer flask; checking the vessels (-> the description of what I see under the microscope is always a delineation of a random specimen I took with the pipet):
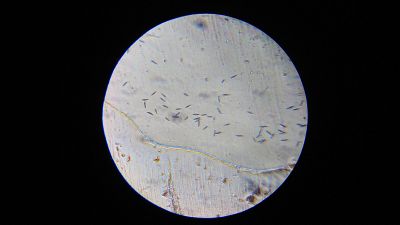
- Tube 1 - a lot of microorganisms; approximately the same amount of Euglena and Paramecia; fast movement (see video below)
- Tube 2 - a lot of microorganisms; more Euglena than Paramecia; fast movement
- Tube 3 - only a few microorganisms; approximately the same amount of Euglena and Paramecia
- Tube 4 - lots of Euglena; few amount of Paramecia
- Erlenmeyer flask - only Paramecia; fast movement
- Beaker glass - only Paramecia; fast movement
Day 5 - The green deposit on the ground of tube 1 grows. Before taking a sample with the pipet, I shake the tube carefully. After about an hour the deposit settles on the ground again.
checking the vessels:
- Tube 1 - only Euglena; high amount; fast movement
- Tube 2 - a lot of microorganisms; much more Euglena than Paramecia; fast movement
- Tube 3 - only a few microorganisms; approximately the same amount of Euglena and Paramecia
- Tube 4 - only a few microorganisms; more Euglena than Paramecia; slower movement than in the other tubes
- Erlenmeyer flask - only Paramecia; fast movement
- Beaker glass - a lot of Paramecia; few amount of Euglena; fast movement
Day 7 - recognizing a green deposit on the ground of tube 2 as well; checking the vessels:
- Tube 1 - only Euglena; high amount; fast movement (see video below)
- Tube 2 - only Euglena; high amount; fast movement
- Tube 3 - approximately five single Euglena
- Tube 4 - only a few microorganisms; more Euglena than Paramecia; slower movement than in the other tubes
- Erlenmeyer flask - few amount of Paramecia; approximately five single Euglena
- Beaker glass - a lot of Paramecia; few amount of Euglena; fast movement
Day 9 - tube 2 (on the left, see photo below) becomes slowly greenish; checking the vessels:
- Tube 1 - only Euglena; high amount
- Tube 2 - high amount of Euglena; approximately two single Paramecia
- Tube 3 - approximately ten single Euglena
- Tube 4 - few amount of Euglena, but more than in the Erlenmeyer flask and beaker glass
- Erlenmeyer flask - few amount of Paramecia; fast movement
- Beaker glass - a few microorganisms; approximately the same amount of Euglena and Paramecia
Day 11 - adding CO2 to tube 3 and the Erlenmeyer flask; checking the vessels:
- Tube 1 - high amount of Euglena (more than in tube 2); fast movement
- Tube 2 - high amount of Euglena; fast movement
- Tube 3 - few amount of Euglena
- Tube 4 - few amount of Euglena, but more than in the Erlenmeyer flask and beaker glass
- Erlenmeyer flask - few amount of microorganisms; much more Euglena than Paramecia
- Beaker glass - a few microorganisms; approximately the same amount of Euglena and Paramecia
Day 13 - checking the vessels:
- Tube 1 - high amount of Euglena (more than in tube 2); fast movement; medium is significantly greenish
- Tube 2 - high amount of Euglena; fast movement; medium is a bit greenish
- Tube 3 - approximately ten single Euglena and three Paramecia
- Tube 4 - few amount of microorganisms; significantly more Euglena than Paramecia
- Erlenmeyer flask - few amount of microorganisms; significantly more Euglena than Paramecia
- Beaker glass - few amount of microorganisms; significantly more Euglena than Paramecia
Day 15 - checking the vessels:
- Tube 1 - very high amount of Euglena; fast movement
- Tube 2 - high amount of Euglena; fast movement
- Tube 3 - small amount of Euglena
- Tube 4 - high amount of Euglena
- Erlenmeyer flask - few amount of Euglena
- Beaker glass - amount of Euglena is significantly growing; only a few Paramecia
Note:
The concentration of Euglena is already visible.
Tube 1: The cheese seems to be magnetic. The area around the cheese is dark green, the medium around it is light green. As soon as I shake it the whole medium in the tube becomes light green
Tube 2: This tube only includes liquid fertilizer. There is no clear concentration point. The medium on the bottom is more green than on the top.
Tube 4: I never shaked this tube before taking a specimen because it has no cover. This time I stired it gently. Compared to the other times I observed it under the microscope, the amount of Euglena was significantly higher. That showed me that the highest concentration of organisms must be on the bottom of the tube. Probably because the cheese always sinks.
Also, whenever I take a specimen, put it on the specimen slide an wait a few minutes, the Euglena start to pile on the fringes of the medium. The middle turns into an an empty space.
Day 17 - checking the vessels:
- Tube 1 - very high amount of Euglena
- Tube 2 - high amount of Euglena; fast movement
- Tube 3 - only a couple of Euglena
- Tube 4 - moderate amount of Euglena
- Erlenmeyer flask - moderate amount of Euglena; a few Paramecia
- Beaker glass - small amount of Euglena; a few Paramecia
Day 19 - checking the vessels:
- Tube 1 - very high amount of Euglena; fast movement
- Tube 2 - high amount of Euglena; fast movement
- Tube 3 - light green deposit on the ground; small amount of Euglena
- Tube 4 - moderate amount of Euglena
- Erlenmeyer flask - small amount of Euglena
- Beaker glass - small amount of Euglena
Day 21 - adding CO2 to tube 3 and the Erlenmeyer flask; checking the vessels:
- Tube 1 - very high amount of Euglena; fast movement
- Tube 2 - high amount of Euglena; fast movement
- Tube 3 - moderate amount of Euglena; fast movement
- Tube 4 - moderate amount of Euglena; fast movement
- Erlenmeyer flask - cheese on the bottom starts becoming green; moderate amount of Euglena
- Beaker glass - cheese on the bottom starts becoming green; small amount of Euglena
Day 23 - adding CO2 to tube 3 and the Erlenmeyer flask; checking the vessels:
- Tube 1 - very high amount of Euglena (I didn't shooke the tube before taking a specimen this time. So I can be sure now that concentration is not even very high on the bottom but also in the whole tube.)
- Tube 2 - high amount of Euglena; fast movement
- Tube 3 - high amount of Euglena; fast movement
- Tube 4 - high amount of Euglena; fast movement
- Erlenmeyer flask - moderate amount of Euglena; fast movement
- Beaker glass - moderate amount of Euglena; fast movement
Note: The medium in the Erlenmeyer flask, the Beaker glass and tube 4 finally starts to become greenish. The cheese on the bottom is dark green but Euglena don't seem to concentrate on the bottom in those vessels. The reason why the organisms grow so fast now is maybe that they increase by splitting up theirselfes. That means that the multiplication would be exponential.
The Paramecia disappeared completely.
Day 25 - checking the vessels:
- Tube 1 - very high amount of Euglena
- Tube 2 - high amount of Euglena
- Tube 3 - high amount of Euglena; fast movement
- Tube 4 - high amount of Euglena; fast movement
- Erlenmeyer flask - moderate amount of Euglena; fast movement
- Beaker glass - moderate amount of Euglena; fast movement
Day 27 - checking the vessels:
- Tube 1 - very high amount of Euglena
- Tube 2 - medium amount of Euglena; fast movement
- Tube 3 - high amount of Euglena; fast movement
- Tube 4 - high amount of Euglena; fast movement
- Erlenmeyer flask - high amount of Euglena; fast movement
- Beaker glass - moderate amount of Euglena; fast movement
Day 29 - checking the vessels:
- Tube 1 - very high amount of Euglena
- Tube 2 - high amount of Euglena; fast movement
- Tube 3 - high amount of Euglena; fast movement
- Tube 4 - high amount of Euglena; fast movement
- Erlenmeyer flask - high amount of Euglena; fast movement
- Beaker glass - moderate amount of Euglena; fast movement
Day 31 - checking the vessels:
- Tube 1 - very high amount of Euglena
- Tube 2 - high amount of Euglena; fast movement
- Tube 3 - high amount of Euglena
- Tube 4 - high amount of Euglena; fast movement
- Erlenmeyer flask - high amount of Euglena; fast movement
- Beaker glass - high amount of Euglena; fast movement
After nearly one month of cultivating Euglena the color of all cultures is finally green. Time to sum up the results.
One of the most important things to say is that in every vessel the Euglena increased to a preferable amount, independently from the ingredients I used for the medium. The big difference is the time they needed.
Tube 1 and 2 were very fast because I used the medium I already cooked what means they have had two weeks to grow before I startet Experiment 1. So I was already able to recognize a medium amount of organisms after three days.
Nevertheless I am convinced that the cheese and the fertilizer helped the growing because of some reasons: The concentration was nearly the same in Tube 1 and 2 and in the Erlenmeyer flask after 30 days. That means the growing in the flask speeded up so that in the end it reached the same concentration with a significantly smaller amount of Euglena in the beginning. Also, the tubes only contain 15ml and the Erlenmeyer flask 100ml - another sign that also without the chemicals a high amount of Euglena can be bred in a short time.
Also I guess that it took longer in tube 3 and 4 because they contained the fewest nutrients. I used cheese and fertilizer but the water was distilled.
There was no noticeable difference in the vessels in which I additionally put CO2 fertilizer once a week.
My recommendation for growing Euglena:
Miga’s recipe (pdf) in combination with a small piece of hard cheese, a warm and light place but no direct sunlight, at least three weeks time and some patience :)
Note: I don’t think the extra solution 3 of the medium is needed anymore because the cheese contains Vitamin B12. To substitute Vitamin B1 some wheat grain should be added as well. I often read that this I used for breeding Euglena too, for example here.
Experiment 2
How does Euglena react to colors?
First attempt
Setup
After nearly one month of cultivating Euglena it is time to start the next experiment. The medium is finally greenish enough to work with it. I filled a tube with the Euglena medium from tube 1 (capacity: 15ml) and filtered all of the cheese out with a sieve. I did this because I guess that it attracts the organisms too much, so that they will swim to the cheese instead of the light. To make the light colorful I cut out stripes of transparent paper and wrapped them around the tube. I chose the fundamental colors yellow, red and blue for the first attempt. To let no "normal" light on the bottom of the tube in, I also prepared a cover made of black nontransparent paper. To not move the tubes when I put off the paper, I put them in my Euglena Workstation to keep them safe. So I can easily remove the paper without mixing the concentrations accidentally. I placed the whole installation in front of a daylight lamp.
Expectation
I guess that the concentration will be very low behind the red and blue paper. As I read during my researches, Euglenas don’t react to red light. Blue is a pretty dark color and I think it won’t attract the organisms much. The yellow paper seems to be the most transparent, so my assumption is that the concentration will be the highest there.
Outcome
After two hours of illumination I removed the papers from the tube. The only remarkable change is that the whole Euglena concentration sank to the ground again. This is strange because this part was covered with dark nontransparent paper. By looking closer it is conspicuous that in the medium are very tiny pieces of decomposed cheese. Because of their tiny size I won't be able to filter them out.
Second Attempt
For the next try I decided to use the aquarium installation instead of a tube. I made good experiences in Experiment 3 (please look below for more information) because the Euglena reacted very sensitive to the light source and did not settle on the ground.
After using the aquarium for experiment 3 and finishing it I pulled a new mask over the glass. It consists of a black nontransparent back and three transparent foils at the front. I put the installation in front of the daylight lamp again and waited for two hours.
After removing the mask I noticed that nothing has changed a all. The "Euglena" writing was still visible. I took a specimen to check under the microscope if the Euglena were okay. To my surprise the organisms were alive and moved. So this phenomenom must have another reason. I can imagine that this happend because the yellow foil was right over the letters. Because this was the lightest part in the installation the organisms had no reason to move away.
Third Attempt
I repeated the experiment with different colors and and a new medium. This time I used the Euglena from the beaker glass. With altogether five colors I wanted to create more options to choose for the organisms.
After one and a half hour of illumination I reached a more satisfying result then before. On the bottom there were clearly visible three separeted stripes. As I knew Euglena is not able to see red light and maybe also orange because it is a mixture of yellow and red. All the other colors - yellow, green and blue - seem to be transparent and light enough to be a good concentration point. Between those stripes were thin parts where the colors overlapped and made a darker shade. Those areas are clear and not (or only less) besieged by the organisms.
I also noticed a green settlement on the ground again. It seems like a part of the concentration always sinks to the bottom of the vessel.
Experiment 3
Can I control Euglena's movement?
First attempt
Setup
I took two pieces of glass (10x15cm) and glued them together on three of four sides. In my first try I used sealing glue but it came out that it was not watertight. So I tried again with silicone. Between the two glass plates there is a small gap of about 2mm. I rinsed the vessel out with distilled water and filled the Euglena medium from the Erlenmeyer flask in the gap. It is conspicuous that the medium does not seem to be green but very clear in this vessel, because the amount is so small. The whole installation is covered with dark, nontranspartent paper on both sides. In the paper I cut some small shapes. I put the installation in front of a daylight lamp.
Expactation
Because Euglena move to light sources the concentration should be remarkable higher in the areas where the vessel is not covered with dark paper.
Outcome
After two hours I removed the paper from the installation. Luckily the experiment was successful and there is actually a clear copy of the letters I cut in the paper. Next time I am going to figure out which camera angle and backround I need to take a better photo. I also want to use a different motif.
Second attempt
I repeated the experiment on the next day. Over night the organisms scattered again so the lettering disappeared. This time I only waited an hour. The result was the same. Euglena seem to be very fast when it is about reaching the light source.
Developing an Euglena Experiment Kit
Experimenting with Euglena in different ways made me even more intersteted in this organism. Especially the experiments with the shapes were very exciting and fascinating. So I decided to develop a toolkit (or the prototype of a toolkit) which makes it easy to arrange similar projects. I still have more ideas what I could do with the tools I have and I am curious what kind of ideas other people have regarding this topic.
Remark: I developed this experiment kit based on the knowledge of Euglena I have by now. The main focus on this project is giving interested people the chance to experiment with Euglena artistically on a low budget.
What is it? A playful installation which makes it possible to draw with the single-celled organism Euglena. If you have different ideas to enhance it - go for it!
What can you do with it? The installation is made for experimenting with Euglena in connection with light, colors and shapes. People who are interested can use it for playing around and turn their pictures into a very different form of drawing. I think it is especially interesting because you can control the development of the outcome not completely. At some point you need to give up the control over your artwork and let Euglena surprise you. You can draw your very own picture, convert it into a mask and receive an Euglena based drawing as outcome. By taking a photo before the artwork fades, you can create a visual memory which can also be printed (e.g. on linen) as art print.
- work in progress -
This is my intermediate result of the toolkit guidance handbook, completion and and proofreading still due.