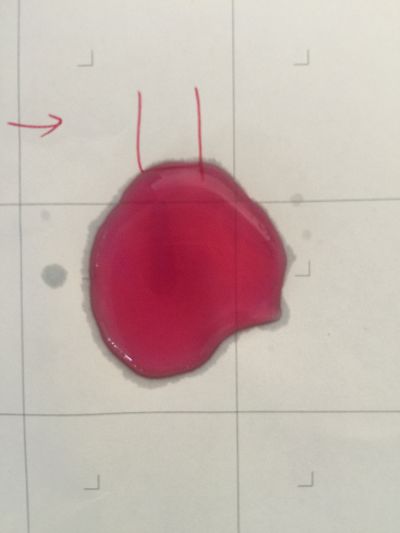
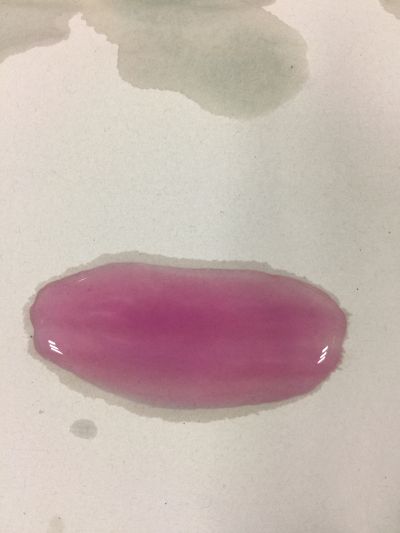
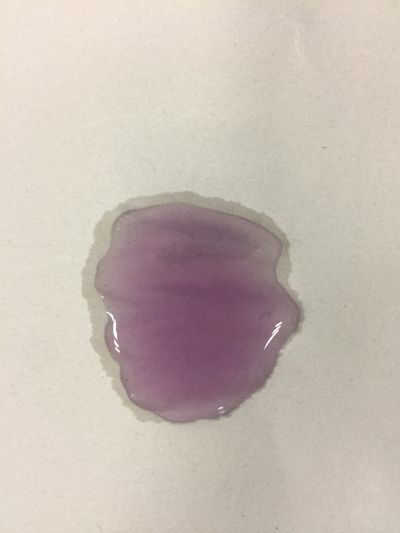
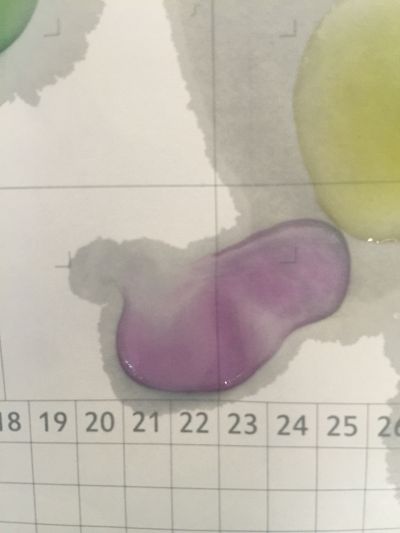
The food we eat every day has gone through a series of procedures before it ever reaches our stomachs. From the beginning of planting, fertilizing, harvesting, to transporting, distributing, and cooking, each procedure involves numerous engineering works and scientific knowledge, each has contributed to the final quality of the food, and each has contributed to the final health of our body. What I focus is when food or drinks mixed with each other, is it still the original one? We drink coffee, mixed milk and sugar or curd. Many people have juicer, mixed kinds of fruits together, may be it has a good flavour, but is it really goo for your health? As we known, acid/Alkaline is very important to our health and alkaline environment is good to our health, how can we know the pH of our drinks in a safely, readable, even edible way? I want to use red cabbage extract solution to know pH of different drinks, I can see changes of colour directly based on the chemical reaction and though compare with pre-testing I have done, I can know pH of drinks easily. The sketch of of pH-testing is
However, there’s a problem which I’m not sure: If pH have directly relationship with health. I read some report and paper, there is a view: alkaline body environment is good for health, but it can not be said alkaline food leads to alkaline environment, it is complex medical problem. And also, this is a website of Acid/Alkaline Food Chart: http://www.phmiracleliving.com/t-food-chart.aspx And I find some healthy medicines which are used to adjust pH, the mainly ingredients are moderately or highly alkaline food. I am confused with it.
References
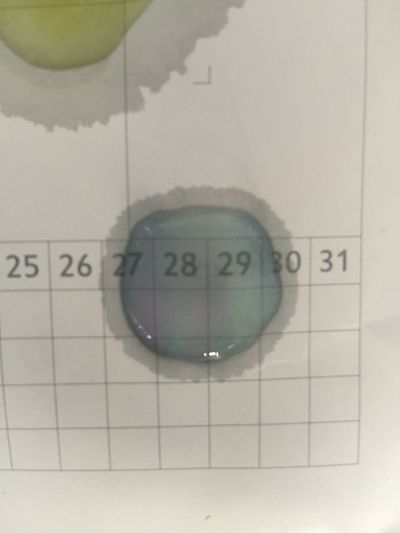
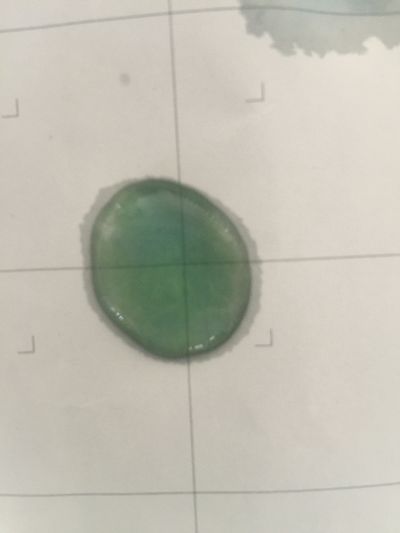
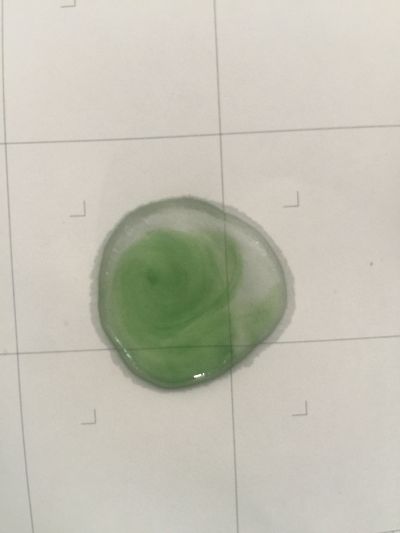
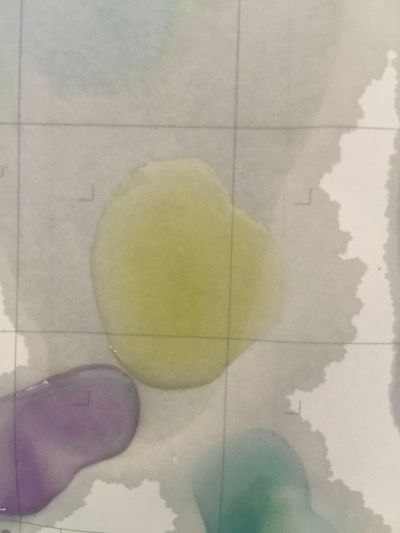
I use red cabbage to make pH indicator. The pH solution from 2 to 13.
The red cabbage solution
1. pH=2 100ml distilled water + 1ml HCL
2. pH=4 100ml distilled water + 0.1g apple acid
3. pH=5.5 100ml distilled water + 2g H3BO3(Boric acid)
4. pH=7 100ml distilled water
5. pH=8 100ml distilled water + 0.5g NaHCO3
6. pH=11 700ml distilled water + 1ml NaOH
7. pH=12 250ml distilled water + 1ml NaOH
8. pH=13 100ml distilled water + 1ml NaOH
after 5 minutes change to yellow
Furthur steps:
1. Rate of colour change
2. Reversibility of colour change
3. Using different pH level solutions react with the organic stuff (which has anthocyanin), see the result