Using red cabbage solution as an indicator to check coffee pH level
1. Introduction
1.1 anthocyanin
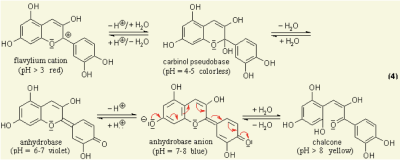
Anthocyanins are water-soluble vacuolar pigments. In the 1664 book Experiments and Considerations Touching Colours by chemist Robert Boyle, various edible plants are reported as visual pH indicators due to pH-responsive mechanisms in their tissues [1]. Anthocyanin is a kind of natural colorant in food and beverage industry, and has been found to possess anti-inflammatory antioxidant properties [2]. It has also been researched for use as an indicator for packaging applications to detect spoilage in pork and fish products [3]. All tissues of vascular plants contain the flavonoid anthocyanin, a pigment that changes colour under varying pH solutions. Under different pH conditions, the hydroxyl (OH) and/or methyl ether (O-CH3) groups attached to the carbon rings (figure 1) undergo reversible structural transformations and ionizations. Restructuring a molecule changes the way it ab- sorbs light, giving rise to colour changes [4].
Figure 1 Chemical diagram of colour-changing anthocyanin pH reaction [5]
1.2 red cabbage
"Red cabbage is rich in a number of bioactive substances, including anthocyanins"[Wiczkowski, 2012]. The method of extracting anthocyanin from red cabbage is easy and convenient.
2. Idea
Coffee always mixed with milk and sugar or curd. We know coffee is a kind of acid drink. When it mixed with water the pH level will change. I want make its pH readable through my experiment.
My first idea is using this chemical reaction as pigments. Like watering the plant which has anthocyanin with different pH solutions to enhance the aesthetic environment. People can monitor and alter the pH of plants to manipulate the way they grow. And I can draw a picture using different pH solutions then spray red cabbage solution on it, colour will change because of the chemical reaction. After discussing with Miga, I think it is a good idea to use red cabbage solution to use red cabbage extract solution to know pH of different drinks and maybe can judge wether it is good to drink.
The food we eat every day has gone through a series of procedures before it ever reaches our stomachs. From the beginning of planting, fertilizing, harvesting, to transporting, distributing, and cooking, each procedure involves numerous engineering works and scientific knowledge, each has contributed to the final quality of the food, and each has contributed to the final health of our body. What I focus is when food or drinks mixed with each other, is it still the original one?
We drink coffee, mixed milk and sugar or curd. Many people have juicer, mixed kinds of fruits together, maybe it has a good flavour, but is it really good for your health? As we know, acid/basic is very important to our health and the alkaline environment is good to our health, how can we know the pH of our drinks in a safe, readable, even edible way? I want to use red cabbage extract solution to know pH of different drinks, I can see changes of colour directly based on the chemical reaction and though comparing with pre-testing I have done, I can know pH of drinks easily. The sketch of pH-testing is
3. Methodology
3.1. Making red cabbage indicator
3.2. Making pH solutions
12.12.2017
3.3. Process of experiment
17.1.2018
However, there’s a problem which I’m not sure: If pH has a direct relationship with health. I read some report and paper, there is a view: alkaline body environment is good for health, but it can not be said alkaline food leads to the alkaline environment/condition, it is complex medical problem. And also, this is a website of Acid/Alkaline Food Chart: http://www.phmiracleliving.com/t-food-chart.aspx And I find some healthy medicines which are used to adjust pH, the main ingredients are moderately or highly alkaline food.
What I will do on 17th:
1. test just for visual and take picture:orange/lemon/onion/green onion/potato...
2. help to know pH level of drinkings
original pH
mixed pH (milk/coffee/tea/gouqi/sugar) (And is there any pH difference belongs to temperature? hot and cold )
question: how to deal with the thing insoluble in water, for instance, oil, cosmetic...
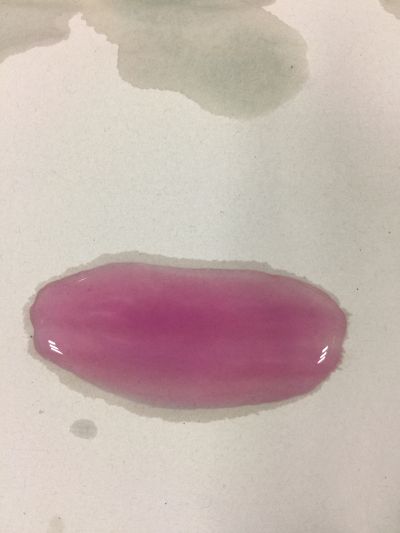
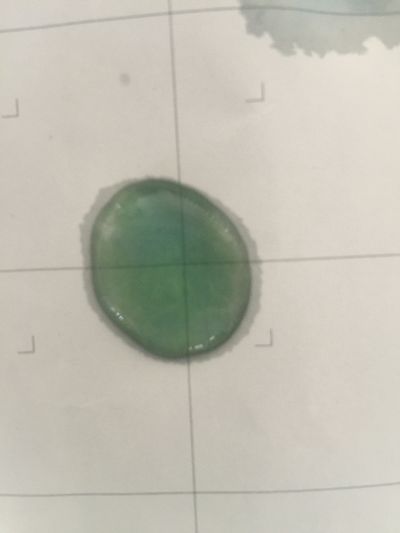
After the work of 17th, I decided to focus on coffee not kinds of drinks to do the experiment. Because it is hard to judge pH level with one colour measurement. I made 3 solutions with different proportions of red cabbage, distilled water and expresso coffee.
1. 5ml red cabbage solution + (1ml distilled water + 1ml coffee)
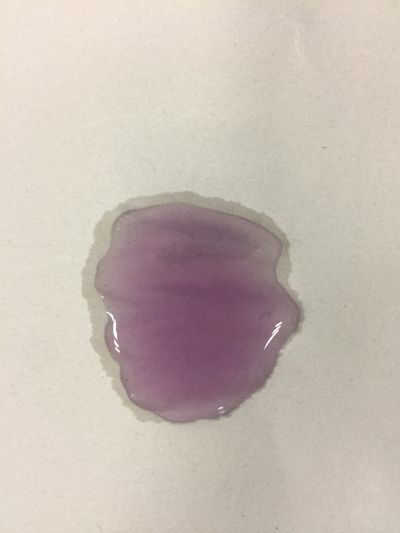
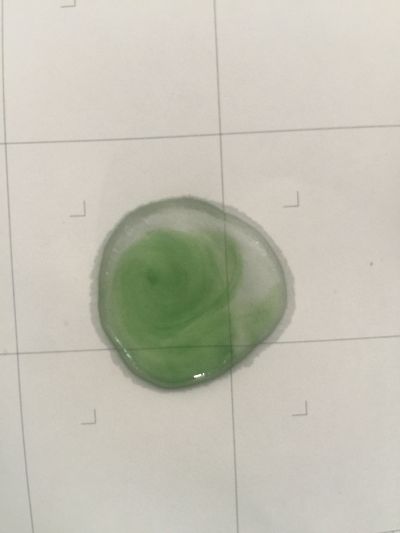
2. 5ml red cabbage solution + (0.5ml distilled water + 1.5ml coffee)
3. 5ml red cabbage solution + (1.5ml distilled water + 0.5ml coffee)
As you can see on follow picture, the coulour results are not obvious. The
19.1.2018
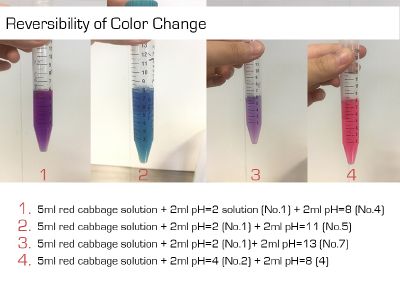
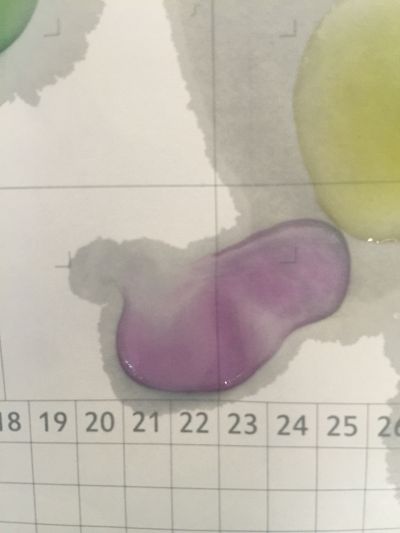
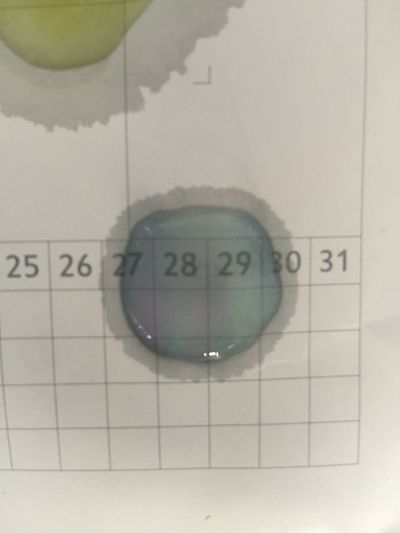
I did the experiment of reversibility of colour change.
And then I use reversibility of colour change to make the colour of coffee more obviously. pH of expresso coffee is around 6, I add the solution (pH=8) into the original solution 1/2/3, the colour changing is more obviously.
4. Discussion
The colour changing is very obviously when drop red cabbage solution into different pH solutions which are coloured transparent. However, coffee is dark, when drop red cabbage solution directly, it is hard to see the colour difference. I use the result of reversibility of colour change, add pH=8 solution into the original coffee and obtain the obviously changing.
5. References
[1] Robert Boyle. 1664. Experiments and Considerations Touching Colours. Project Gutenberg. 1–157 pages.
[2] Jian He and M Monica Giusti. 2010. Anthocyanins: Natural Colorants with Health-Promoting Properties.
[3] Xiahong Zhang, Sisi Lu, and Xi Chen. 2014. A visual pH sensing film using natural dyes from Bauhinia blakeana Dunn. Sensors and Actuators B: Chemical 198 (2014), 268–273.
[4] Viirj Kan. 2017. Organic Primitives: Synthesis and Design of pH-Reactive Materials using Molecular I/O for Sensing, Actuation, and Interaction. Proceedings of the 2017 CHI Conference on Human Factors in Computing Systems Pages 989-1000.
[5] UMass Amherst Department of Chemistry Lecture Demonstrations.
File:12.6 bio-lab first idea.pdf
12.12.2017
I use red cabbage to make pH indicator. The pH solution from 2 to 13.
The red cabbage solution
1. pH=2 100ml distilled water + 1ml HCL
2. pH=4 100ml distilled water + 0.1g apple acid
3. pH=5.5 100ml distilled water + 2g H3BO3(Boric acid)
4. pH=7 100ml distilled water
5. pH=8 100ml distilled water + 0.5g NaHCO3
6. pH=11 700ml distilled water + 1ml NaOH
7. pH=12 250ml distilled water + 1ml NaOH
8. pH=13 100ml distilled water + 1ml NaOH
after 5 minutes change to yellow
Furthur steps:
1. Rate of colour change
2. Reversibility of colour change
3. Using different pH level solutions react with the organic stuff (which has anthocyanin), see the result
However, when I change another kind of coffee and do the experiment in the same way, the colour change cannot be seen obviously. Maybe because the different ingredients of coffee.